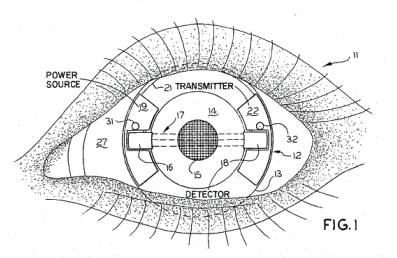
Here is an early patent for determining blood glucose levels by looking into your eye. U.S. Patent 3,958,560 Non-Invasive Automatic Glucose Sensor System issued May 25, 1976 to Wayne Front March.
Jed Margolin
Introduction
There have been two methods used for home testing of glucose levels: urine dipsticks (which are of limited value) and blood analysis (which requires that you stick yourself).
We want something that is accurate as well as non or minimally invasive.
There is currently work being done using interstitial fluid, infrared spectroscopy, radio frequency signatures, ultrasound, and observation of the fluid in the eye.
I propose a method of determining blood glucose levels through the analysis of exhaled breath. I don't know exactly how to do it, yet. So if all you are interested in is the solution you might as well stop reading now.
But it is a product well worth going for.
In 2002 the market for blood glucose meters was $252.8 million and the market
for blood glucose test strips was $1.132 billion.
Background
Here is a simplified explanation of how things work
Everything you eat is converted into glucose which ends up in your blood. It is glucose that powers your cells. However, the cells do not use glucose directly. The walls of most of your cells contain receptors for glucose and for insulin (among many other things). Once through the cell wall, glucose and (usually) oxygen are involved in a complicated multistep process and turn into Adenosine Triphosphate (ATP) which is what directly powers the cells.
There are some cells that do not require insulin for efficient uptake of glucose; important examples are the brain and the liver.
If you don't have enough insulin, the cells that need it don't get the ATP they need. And since glucose then builds up in the blood, the cells that don't use insulin to convert glucose into ATP get too much glucose.
Normally, your pancreas produces insulin (a hormone) in response to blood glucose levels. In diabetics the pancreas doesn't produce enough insulin; it might not produce any.
By the way, there is a substance in your body called Plasma Cell Membrane Glycoprotein-1 (PC-1) which competes with insulin at the cell wall receptors. (While the amount of PC-1 you have is heavily dependent on heredity, the fatter you are, the more PC-1 you have.) When PC-1 inhibits the insulin receptors, the glucose doesn't get through the cell walls to be converted into ATP.
When your cells don't get the ATP they want they start yelling, “Feed Me,” so you eat more. Eventually the glucose you eat does get converted into ATP but the excess calories go into the fat cells, which therefore results in your body having more PC-1 and you want to eat even more.
I suspect that there are other hormones produced by the body that compete with insulin. In particular, how about the hormones produced when you are under stress? That would explain why many people eat when they are under stress. Their cells aren't getting the ATP they need and are screaming (again) “Feed Me.”
There are around 13 million people in the United States who are diabetic and maybe another 5 million who are diabetic and don't know it (yet). [American Diabetes Association: http://diabetes.org/about-diabetes.jsp ] Estimates vary.
The primary method of measuring blood glucose levels requires a sample of blood. (Glucose does not even start to appear in the urine until blood glucose levels are already dangerously high, about 180 mg/dL.)
When done by a health care professional a blood glucose test is an expensive procedure. It is also likely to be done at infrequent intervals.
While it can easily be done at home using one of a number of home test kits, it still requires getting a blood sample which is a real barrier for most people even though it only requires sticking your finger. People who do not suspect they are diabetic (or heading in that direction) are not likely to buy and use a home test kit that requires that they stick themselves and draw blood.
That's unfortunate, because blood glucose levels can provide useful information even in a non-diabetic. For example, an infection commonly raises blood glucose levels. So does stress. Having an elevated blood glucose level can alert you to take better care of yourself. It can even give you “permission” to slow down.
Here are some useful links to cell metabolism:
1. Cellular Respiration Summary
http://www.ucalgary.ca/~rosenber/CellularRespirationSummary.html
2. Biomedical Hypertextbooks from Colorado
State University - Physiologic Effects of Insulin
http://arbl.cvmbs.colostate.edu/hbooks/pathphys/endocrine/pancreas/insulin_phys.html
3. Energy Metabolism in Cells
http://campus.northpark.edu/biology/cell/energymetab.html
4. Insulin and Glucagon
http://www.medbio.info/Horn/Time%203-4/homeostasis_2.htm
Prior Art – History of Home Glucose Testing
Home glucose testing became practical in the late 1950s with the development of urine dipsticks. A chemical reaction caused the dipstick to change color according to the glucose concentration in the urine. The user was then required to visually compare the intensity of color with a reference color chart to arrive at a value. While simple to use, these devices were inconvenient and lacked the precision necessary for insulin dosage adjustments. Accurate and precise measurement of blood glucose levels could be achieved only with laboratory instruments.
An example of this technology is U.S. Patent 2,893,843 Composition of Matter issued July 7, 1959, to Ernest C. Adams Jr. (Miles Laboratories) which appears to be the first patent for a urine test strip that could be used by regular people at home.
According to the patent (Column 1, lines 17-21):
This invention relates to a novel method and means for the detection and estimation of glucose.My invention has for one of its principal objects the provision of a simple, rapid, and convenient means for performing such a test with a high degree of accuracy and simplicity without the need for extensive equipment or trained personnel and the like.
The way it works is (from Column 1,
line 58 - Column 2, line 4):
My invention operates on a new and different principle and takes advantage of the fact that in an aerobic system, in the presence of glucose aerodehydrogenase (glucose oxidase), glucose is oxidized to gluconic acid and hydrogen peroxide. This reaction is highly specific for glucose, other sugars being oxidized only to an infinitesimal extent.The resulting hydrogen peroxide can be detected in any one of a number of ways by a color given with, for example, blood, or certain components thereof, and a suitable indicator such as o-tolidine, benzidine and other members of the group represented by those indicators, as well as leuco-dyes, monoamines, diamines, Phenols, diphenols, aromatic acids and iodides.
Although the patent clearly mentions
being usable for testing blood, its main use appears to have been to test
urine.
You can read the
complete 2,893,843 patent here (306 KBytes PDF).
From the LifeScan web site A BRIEF HISTORY
OF BLOOD GLUCOSE MONITORING
http://www.lifescan.com/pdf/hospital/eb_110a.pdf.
In the late 1970s, similar test strips for measuring glucose in whole blood samples were introduced. Like the earlier urine dipsticks, the whole blood test strips used a reagent-impregnated pad on a plastic strip to enzymatically convert glucose to a colored product. Proper use of the whole blood test strips required removal of excess sample (by wiping or blotting), careful timing, and visual estimation of glucose concentration; the quality of results was highly operator dependent.
An early patent (maybe the first) for
making test strips for specifically testing blood is U.S. Patent 3,061,523
Method
For Determining Glucose in Blood issued October 30, 1962, to Alfred
H. Free (Miles Laboratories).
From Column 1, lines 30 - 48:
There are a number of methods, of course, which can be used to measure or estimate the amount of glucose in blood. The more widely used of the conventional are based on the use of alkaline copper solutions which are heated with the materials being tested to precipitate cuprous oxide.The old methods have had the disadvantage that their use has required a certain amount of skill and familiarity with the use of measuring equipment such as pipettes and the like, the use of liquid reagents some of which, especially the alkaline ones, were dangerous to handle and inconvenient to transport easily,
However, all of these tests, techniques, and procedures have as characteristics in common the need for heat generally supplied by some extraneous source like a Bunsen burner to carry out the tests, and also require a test tube or like container within which the testing is to take place. Some of these tests additionally are impractically time consuming.
Here's what he does (Column 1, line
58 - 65):
In practicing my invention, I prepare a composition of glucose-oxidase, peroxidase, an indicator whose color is affected by hydrogen peroxide in the presence of peroxidase, and in addition to the foregoing and desirably, a buffer to keep the pH of the reactants at the site of reaction within a predetermined range, a stabilizer such as gelatin or similar material, and in certain situations a dye to make color reading easier.You can read the complete 3,061,523 patent here (257 KBytes PDF).
[Continuing from LifeScan]:
Shortly after the introduction of visually read test strips, the first generation of hand-held blood glucose meters reached the market. By incorporating a small optical system (a reflectance photometer), these meters allowed the user to read the test strips electronically to measure the color intensity on the test strips. Optical readings were converted to a digital display of blood glucose concentration. Although such systems still required wiping and timing, their enhanced performance allowed people with diabetes to accurately and precisely self-monitor their blood glucose levels.Miles Laboratories was again the pioneer.
You can read the
complete 3,604,815 patent here (821 KBytes PDF).
The history of Miles Laboratories is very interesting. In addition to its work in glucose testing, Miles was also the creator of Alka Seltzer, One-A-Day Vitamins, and Flintstones chewable children's vitamins. The company’s history can be found at: http://www.bayerus.com/communities/elkhart/about/history.html
In 1978 Miles Laboratories was bought by
Bayer AG of Leverkusen, Germany. (At the time it was in West Germany; now
it’s just Germany again.) In 1994 Bayer purchased the North American over-the-counter
drug business of Sterling Winthrop, acquiring the Bayer Aspirin product
line and the North American rights to the Bayer name and trademarks. Sterling
Products had obtained the property in auction after it was confiscated
by the U.S. government in 1918, following the end of World War I.
U.S. Patent 4,509,859 Apparatus for Optoelectronic Evaluation of Test Strips Issued April 9, 1985 to Markart Ernst, Blumel Reinhart, and Eggert Holgar is an early attempt to deal with the problems of calibrating the test meters to account for, among other things, the nonuniformity of the batches of chemicals used in the test strips. It includes the use of a bar code reader setting the calibration of the meter to the particular batch of test strips.
You can read the
complete 4,509,859 patent here (435 KBytes PDF).
[Continuing from LifeScan]:
Second-generation meters for SMBG reached the market in the mid to late 1980s. These meters eliminated the need for wiping and timing. A second technology – electrochemistry - joined reflectance photometry for blood glucose monitoring. Both technologies use an enzyme to convert glucose to a measurable product, but instead of measuring a colored product, electrochemical meters quantify the number of electrons generated by the enzymatic reaction and convert that number to blood glucose concentration.Here is LifeScan's patent using reflectance photometry: U.S. Patent 4,935,346 Minimum Procedure System for the Determination of Analytes issued June 19, 1990 to Roger Phillips, Geoffery McGarraugh, Frank Jurik, and Ray Underwood.
You can read the complete 4,935,346 patent here (1.5 MBytes PDF).Abstract A method for determining the presence of an analyte in a fluid is described along with various components of an apparatus specifically designed to carry out the method. The method involves taking a reflectance reading from one surface of an inert porous matrix impregnated with a reagent that will interact with the analyte to produce a light-absorbing reaction product when the fluid being analyzed is applied to another surface and migrates through the matrix to the surface being read. Reflectance measurements are made at two separate wavelengths in order to eliminate interferences, and a timing circuit is triggered by an initial decrease in reflectance by the wetting of the surface whose reflectance is being measured by the fluid which passes through the inert matrix. The method and apparatus are particularly suitable for the measurement of glucose levels in blood without requiring separation of red blood cells from serum or plasma.
Although Miles Laboratories was the pioneer in home glucose testing they lost their dominance of the market to an upstart: LifeScan of Milpitas, CA. LifeScan was later bought by Johnson & Johnson for an estimated $100M. In medical devices, as in most industries these days, innovations are made by small companies who are then gobbled up by big companies.
From an article on blood glucose meters by journalist Rick Mendoza at http://www.mendosa.com/meters.htm:
The U.S. market for blood glucose meters was worth $252.8 million in the year ended March 31, 2002, according to the June 2002 issue of OTC Update, published by Nicholas Hall Periodicals. This is up from $224.4 million in 2001 and $195.0 million in 2000.But blood glucose strips are far more important. Sales in 2002 were $1,132.6 million. That's up from $1047.4 in 2001 and $971.1 million in 2000.
LifeScan/J&J still dominates this market with a 44 percent share of meter retail sales and 45 percent of blood glucose strips. Roche Diagnostics is a distant second with 28 percent of meters and 27 percent of strips. Bayer is third with 12 percent of meters and 15 percent of strips. MediSense/Abbott is fourth with 10 percent of meters and strips. All others account for just 6 percent of meters and 3 percent of strips.
Some good references on the history
of home testing are:
1. The history of home testing: http://www.mendosa.com/history.htm
2. An account of home glucose testing can
be found in My First Fifty Years as a Diabetic by Dr. Richard Bernstein
(http://www.diabetes-normalsugars.com/readit/fiftyyears.shtml).
Dr. Bernstein is known for his book Dr. Bernstein’s Diabetes Solution.
Prior Art – Current Research
So far we have discussed two methods: urine dipsticks (which are of limited value) and blood analysis (which requires that you stick yourself).
We want something that is accurate as well as non or minimally invasive.
It is a product well worth going for. In 2002 the market for blood glucose meters was $252.8 million and the market for blood glucose test strips was $1.132 billion. (That’s $1132 million for you Brits.)
From the National Diabetes Information
Clearinghouse (NDIC) web site at:
http://diabetes.niddk.nih.gov/dm/pubs/glucosemonitor/index.htm
Researchers are developing other methods of noninvasive monitoring. Potential ways to determine blood glucose levels include:shining a beam of light onto the skin or through body tissues
measuring the energy waves (infared radiation) emitted by the body
applying radio waves to the fingertips
using ultrasound
checking the thickness (also called the viscosity) of fluids in tissue underneath the skin
The National Diabetes Information Clearinghouse (NDIC) is a service of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). The NIDDK is part of the National Institutes of Health under the U.S. Department of Health and Human Services.
Interstitial Fluids
The Glucowatch is a device that measures glucose level in interstitial fluid.
From http://diabetes.niddk.nih.gov/dm/pubs/glucosemonitor/index.htm
Noninvasive Blood Glucose MonitorsThe U.S. Food and Drug Administration (FDA) has approved a noninvasive blood glucose monitoring device for adults, children, and adolescents with diabetes. Noninvasive monitoring means checking blood glucose levels without puncturing the skin for a blood sample. The GlucoWatch G2 Biographer, manufactured by Cygnus Inc., was approved to detect glucose level trends and track patterns in people with diabetes. It must be used along with conventional blood glucose monitoring of blood samples. The device, which looks like a wristwatch, pulls body fluid from the skin using small electric currents. It can provide six measurements per hour for 13 hours.
From an article on the Glucowatch by
journalist Rick Mendoza (http://www.mendosa.com/glucowatch.htm):
You can wear the GlucoWatch like a wristwatch. It uses a low electric current to pull glucose through the skin, so it is minimally invasive. You will be able to program a built-in alarm to alert you when your glucose level is dangerously low or high.Unfortunately, the Glucowatch appears to be difficult to use; besides which it uses a sensor pad that costs about $7.50 and only lasts about 13 hours.
The Glucowatch is made by Cygnus; their
web site is at http://www.glucowatch.com
Infrared Spectroscopy
There are major efforts going on for using infrared spectroscopy. This one looks very promising.
From http://www.ieee.org/organizations/pubs/newsletters/leos/apr98/midinfrared.htm
Mid-Infrared Spectroscopy for Noninvasive Blood Glucose MonitoringDavid C. Klonoff, MD
Department of Medicine
University of California at San Francisco San Francisco, Californiaand
James Braig, Bernhard Sterling Ph.D., Charles Kramer Ph.D., Daniel Goldberger and Rick Trebino Ph.D.
OptiScan Biomedical Corporation
Alameda, CaliforniaMid-IR spectroscopy is an attractive technology for blood glucose monitoring because it is noninvasive, specific, rapid, and potentially inexpensive. The remainder of this article discusses that methodology.
In the mid-IR, glucose absorbs strongly with minimal interference from other absorbing species, but sources are not ideal and water absorbs very strongly. The OptiScan noninvasive glucose meter takes advantage of the realization that the human body is an excellent blackbody emitter of mid-IR light at precisely the right spectral region, so the necessary radiation is therefore already present inside the body near enough to the surface (within 10’s of microns) that its emission spectrum may be used to measure glucose.
Unfortunately, the nature of blackbody radiation is such that, if the glucose to be measured is at the same temperature as the tissue that emitted the blackbody radiation, then the glucose will be effectively transparent, and no glucose spectrum will be observed in the emitted light due to the balance of absorption, spontaneous emission, and stimulated emission at equilibrium. Fortunately, this problem can be solved by introducing a temperature gradient—e.g., cooling the surface—where the cooler glucose will absorb more than it will emit. This yields a reasonable glucose absorption spectrum superimposed on the usual smooth blackbody emission spectrum.
OptiScan Biomedical Corporation of
Alameda, California already has a number of patents. One is U.S. Patent
6,198,949 Solid-State Non-Invasive Infrared Absorption Spectrometer
for the Generation and Capture of Thermal Gradient Spectra From Living
Tissue issued March 6, 2001, to James R. Braig, Bernhard B. Sterling,
Daniel S. Goldberger, Joan C. Godfrey, Julian Cortella, David J. Correia,
Qrthur M. Shulenberger, and Charles E. Kramer.
You can read the complete 6,198,949 patent here (1.7 MBytes PDF).
A good discussion of the infrared properties of blood can be found in U.S. Patent 5,313,941 Noninvasive Pulsed Infrared Spectrophotometer issued May 24, 1994 to James R. Braig and Daniel S. Goldberger.
You can read the
complete 5,313,941 patent here (1.7 MBytes PDF).
Photoacoustic
There is a company named Glucon which is combining optical and sound-based technology. Although they claim to have a number of pending patents they have not provided any useful details of their method.
From http://www.glucon.com:
The Glucon devices are based on a novel Photoacoustic (optical and sound-based) technology protected by several pending patents, and intended to be incorporated into a small, conveniently wearable instrument (the Personal Device).
The company believes that Photoacoustics,
a unique combinatorial technology, is superior to optics alone, as it attains
blood glucose measurements directly from inside the blood vessels and is
an improved method both for specificity (identification) and sensitivity
(detection of level changes) of the glucose
measurement.
The Eye

Here is an early patent for determining
blood glucose levels by looking into your eye. U.S. Patent 3,958,560 Non-Invasive
Automatic Glucose Sensor System issued May 25, 1976 to Wayne Front
March.
It appears to use a special contact lens containing a transmitter, a detector, and a power source.
You can read the complete 3,958,560 patent here (387 KBytes PDF).
While it might not have been practical
(or even possible) to build this device in 1976, it should be easy to build
today. Note that since the patent was issued in 1976, it would have expired
17 years later, in 1993.
Here is another method using the fluid
in the eye. This one is from the Karlsruhe Universitat Institut für
Technik der Informationsverarbeitung (ITIV) [University of Karlsruhe
Department of Electrical Engineering & Information Technology]:
http://www-itiv.etec.uni-karlsruhe.de/opencms/opencms/en/research/workgroups/MST_Optik/vitalsensorik/glukose.html
Optical measurements are well suited for non-invasive and non-contact measurement systems. Due to its excellent optical qualities the anterior chamber of the human eye is a very interesting target for optical measurements. The contained liquid (aqueous humor) mainly consists of blood serum and hence reflects the blood glucose concentration considering a certain time delay.Personally, if I wanted something implanted in my body, my eye would be the last place I would choose.Meeting the strongly limiting laser safety regulations a light beam can be directed into the anterior chamber which is reflected by the surface of the IOL. The light crosses the anterior chamber twice and the optical properties of the aqueous humor can be analyzed. Using these measurement results the glucose level respectively the blood sugar level can be derived.
.
.
.
Optical measurement principles at the human eye analyzed by several research groups so far have all miscarried due to the same fundamental problem: The extremely small measurement signal i.e. the tiny amount of light which is being reflected by the IOL and holds the valuable information about the glucose concentration. Only a percentage of about 0.05% of the incoming light is reflected by the human IOL. Together with safety regulations which strongly limit the allowed optical power density at the retina, optical measurement techniques at the human anterior chamber are at the edge of feasibility. A very promising option to solve this problem are miniaturized passive implants.Modified Intraocular Lens
As mentioned before a large part of the Type II diabetics are in need of an artificial IOL since their natural lens has become dull and rigid due to aging processes which is why vision is fading continuously (Cataract). Replacement of the natural lens by an artificial IOL is usually done ambulatorily. The minimally invasive operation itself takes about 20 minutes per eye and is done via a cut of only 2mm length.Together with industrial partners ITIV is developing a specialized IOL which will transmit the visible light and strongly reflect the wavelengths of the lasers in use relevant to the measurement.
This way the deficiency of polarimetry as well as spectroscopy can be compensated.
Implantable Passive Sensors
All usable optical effects inside the eye only offer very small signal magnitudes and are hard to detect. While further chemical and physical effects offer much higher magnitudes utilizing such effects seems obvious.A passive implantable miniaturized sensor is being developed at ITIV. Using the same minimal-invasive implantation techniques used in modern IOL implantation such a miniaturized sensor could be easily implanted. For information readout a non-contact optical measurement technique will be employed. Such a sensor will enable diabetics to conduct more frequent, painless and easy-to-do measurements..
But at least I understand why the existing proposed eye scanners have not taken over the market:
The extremely small measurement signal i.e. the tiny amount of light which is being reflected by the IOL and holds the valuable information about the glucose concentration. Only a percentage of about 0.05% of the incoming light is reflected by the human IOL.Perhaps this problem of having small signals could be overcome by the massive use of computing power which is getting ever cheaper even as you read this.
Breath Analysis
The idea came to me one day when I took my car in for its biennial smog test. An internal combustion engine takes in air (containing oxygen and some other gases), combines it with fuel (generally gasoline), causes oxidation to occur (it happens quickly enough that we perceive it as an explosion), converts some of the heat produced into mechanical energy, and blows the products (heat, carbon dioxide, water, and a bunch of other stuff) out the tailpipe.
The human body is a chemical engine that operates in much the same way. We take in air (containing oxygen and some other gases), combine it with fuel (food that is turned into glucose), cause oxidation to occur (relatively slowly), convert the energy into mechanical and chemical energy, and get rid of the waste products through several means, one of which is by exhaling.
By measuring the products that come out when you exhale, it should be possible to get a very good idea of what is going on inside the engine (your body).
Your first thought might be, “ This is a waste of time, glucose is not a blood gas.”
While glucose is not a blood gas, neither is alcohol, and it is certainly possible to measure blood alcohol levels from breath analysis.
From an article Breath Tests in Medicine By Michael Philips (Scientific American July 1992 page 74-79):
All the breath tests described so far detect either a volatile organic compound that has been previously consumed (such as alcohol) or the metabolites of a precursor. Researchers has attempted to advance one step further by detecting the volatile compounds present in the breath under normal circumstances. In 1971 Linus Pauling described an elegantly simple method for microanalysis of normal breath. He passed breath through a cold trap consisting of a stainless-steel tube chilled by dry ice. He then assayed the condensate by gas chromatography and mass spectroscopy. He observed approximately 250 different compounds. This surprisingly large number indicated that the composition of human breath was far more complex than had been previously suspected. Other laboratories have confirmed his findings, and researchers have now isolated nearly 400 volatile organic compounds in normal human breath.Michael Phillips MD, FACP, is the founder and chief executive officer of Menssana Research, Inc. He is also Professor of Clinical Medicine at New York Medical College, Valhalla, New York.
The Scientific American article is available from the Menssana web site at: http://www.menssanaresearch.com/brethmed.pdf
Another interesting article is Breath Tests May Reveal Illnesses (Wall Street Journal October 1, 2003, page D10) available at: http://www.menssanaresearch.com/wsj011003.pdf
The Menssana Research web site is: http://www.menssanaresearch.com/
One of the compounds in human breath is acetone.
U.S. Patent 5,174,959 Breath Component Monitoring Device Issued December 29, 1992, to Samar Kundu, Richard W. George, Steven C March, and Sangvorn Rutnarak (Abbot Laboratories).
Here is why measuring ketones is important. From Column 1, lines 21 – 54:Abstract The present invention relates to methods and materials for the detection of ketone and
aldehyde analytes in fluid samples by means of reacting analyte containing samples with a first solid matrix material to which a nitroprusside salt is coupled and a second solid matrix material to which an amine is covalently coupled. Methods and devices are also provided for ascertaining the fat catabolism effects of a weight loss dietary regimen comprising determining the breath acetone concentration of the subject.
It is known that "ketone bodies" by which term is generally meant acetone, acetoacetic acid and beta.-hydroxybutyric acid, tend to accumulate in the blood stream during periods of relative or absolute carbohydrate deprivation due to the breakdown of storage triglycerides. The process through which overproduction of ketone bodies occurs is not well defined but is related to increased oxidation of long chain fatty acids by the liver. Specifically, acetoacetic acid and .beta.-hydroxybutyric acid are formed by the liver as intermediates during the oxidation of fatty acid molecules by acetoacetyl coenzyme A. Acetone is formed from the spontaneous decarboxylation of acetoacetic acid. Under normal conditions the intermediate products are further degraded to carbon dioxide and water and the ketone products do not appear at significant concentrations in the bloodstream. Nevertheless, certain metabolic and disease states interfere with the normal degradation of these intermediates which then accumulate in the bloodstream as a result.And from Column 7, line 53 – Column 8 line 2:The quantitative measurement of ketone concentrations in blood serum is important because of the relationship between elevated serum ketone levels and clinical conditions such as diabetes, disorders of the digestive organs, renal insufficiency, uremia and malignant carcinoma. In the course of these disorders, ketone bodies pass into the blood stream and a state of metabolic acidosis (ketosis) occurs. Monitoring for the onset of ketosis is of particular importance in the maintenance of diabetics because the occurrence of ketosis may indicate the need for modification of insulin dosage or other disease management.
Of interest to the present invention are observations that ketosis occurs in non-diabetic individuals undergoing weight loss through diet, fasting or exercise. Freund, Metabolism 14, 985-990 (1965) observes that breath acetone concentration increases on "fasting." It is disclosed that breath acetone concentrations increased gradually from the end of the first day of the fast to approximately 50 hours into the fast at which time the concentration rose sharply in a linear fashion and reached a plateau on the fourth day. The acetone concentration of the plateau was approximately 300 .mu.g/liter (5,000 nM) a hundred-fold increase over the normal value of 3 .mu.g/liter (50 nM). When, instead of fasting, the subject was placed on a "ketogenic" diet with a minimum of 92% of calories derived from fat, the subject suffered a lesser degree of ketosis wherein the plateau had an acetone concentration of approximately 150 .mu.g/liter (2,500 nM).
By measuring the ketone blood level
(by measuring breath acetone concentration) you can give people almost
immediate feedback on how well they are sticking to their diet, as opposed
to just weighing yourself which produces poor feedback and tends to cause
people to become discouraged and give up on their diet too soon.
The device described in the patent uses consumables, as would be expected from a pharmaceutical company like Abbot Laboratories. When you’re a hammer, everything looks like a nail.
You can read the
complete 5,174,959 patent here (1.9 MBytes PDF).
A more recent patent that can be used to measure ketones in exhaled breath is U.S. Patent 6,609,068 Personal Computer Breath Analyzer for Health-Related Behavior Modification and Method issued August 19, 2003, to Paul E. Cranley, James D. Tate, Ted E. Miller, Alan D. Strickland, Charles J. McDonald, Michael J. Bartels; Alan K. Schrock, and Scott P. Crane.
This device uses one of a number of solid state sensors now available.
You can read the
complete 6,609,068 patent here (1.5 MBytes PDF).
There has been a first step taken of using breath analysis to measure blood glucose levels: U.S. Patent 6,602,715 13C Glucose Breath Test for the Diagnosis of Diabetic Indications and Monitoring Glycemic Control issued August 5, 2003, to Randall W. Yatscoff, Robert T. Foster, Launa J. Aspeslet, and Richard Lewanczuk (Isotechnika Inc.).
Abstract A breath test and kit for performing the breath test are described for the diagnosis of diabetic indications and monitoring of glycemic control. The breath test utilizes the measurement of expired 13C-labeled CO2 following the ingestion of a 13C-enriched glucose source.
A 13C-enriched glucose
source is a glucose source where some of the carbon is carbon-13 instead
of carbon-12, which is the normal form of carbon. According to the
patent, it is safe since carbon-13 is a naturally occurring isotope found
in all carbon-containing substances and is not radioactive. You eat the
13C-enriched
glucose source, it gets turned into glucose in your blood, then ATP in
the cells, and the products are CO2 and water. When you exhale
the CO2 some of the carbon is the carbon-13 from the 13C-enriched
glucose source, which is why it is called
13C-labeled CO2
.
Breath samples are collected before the test and at regular intervals. The purpose of the test is to see if the person’s response to the glucose is normal or if the person is beginning to exhibit insulin resistance. The breath samples are then sent to a lab for analysis.
The idea is that breathing into a container is a lot easier than having blood taken. (And they’re right.)
You can read the complete 6,602,715 patent here (1.1 MBytes PDF).
This patent is a good start, but we want
to be able to read blood glucose levels whenever we want to, not in response
to eating what amounts to a calibrated meal.
Here are some possibilities:
Look for something already in exhaled breath.
It might be a single substance. It might be a combination of substances or the ratio between two or more substances.
It might require that the user take a calibrated inhaled breath. That’s ok, we can have the user inhale through our device and measure the air being inhaled.
We can add something to the breath being inhaled. However, it has to be something that is completely innocuous and also extremely cheap. Anything radioactive is completely out. Maybe some form of a nitrogen gas compound. Or maybe a form of an oxygen gas compound. Maybe a non-radioactive isotope of oxygen. (“Read your glucose level and get a boost at the same time.”) Hopefully, it won’t be helium.
If we are really lucky it could require
eating something that looks like a small piece of candy that tastes like
chocolate.
Jed Margolin
San Jose, CA
February 24, 2004
|
|
Copyright 2004 Jed Margolin
People Links:
1. Frederick Banting (from PBS) http://www.pbs.org/wgbh/aso/databank/entries/bmbant.html
Frederick Banting began his studies at the University of Toronto with the aim of entering the ministry, but instead he switched to medicine, receiving his MD in 1916. After graduating, he joined the army and served as a medical officer during World War I. He was awarded the Canadian military cross for bravery, attending wounded soldiers even while he himself was wounded. After the war, he practiced medicine in London, Ontario, until 1921, when he and Charles Best began their research into the hormone insulin.The work progressed rapidly from basic research to clinical application and Banting, along with John J.R. Macleod, head of the physiology department at the University of Toronto, were awarded the Nobel Prize in medicine/physiology in 1923. They were the first Canadians to ever receive that honor. Banting initially threatened to refuse the award because he felt Charles Best's work as research assistant had been vital to the project and that he should be included in the honor. Ultimately Banting accepted, and shared his portion of the prize with Best. Later Banting was named head of a new department of medical research at the University of Toronto, named after him and Charles Best. He became Sir Frederick Banting when he was knighted in 1934. On February 21, 1941, Banting was killed in a plane crash while on a military medical mission in Newfoundland.
2. Charles Best (from PBS) http://www.pbs.org/wgbh/aso/databank/entries/bmbest.html
Charles Herbert Best was born in Maine, the son of a physician. He joined the Canadian artillery during World War I, and thus qualified for Canadian citizenship.Best was still a medical student at the University of Toronto when he joined Frederick Banting in his work to isolate the pancreatic hormone insulin and apply it to the treatment of diabetes. The work had personal significance for Best, as his favorite aunt had recently died of the disease. He finished his medical degree in 1925, two years after Banting and physiology professor J.J. Macleod received the Nobel Prize for the work. Banting, who felt that Best should have been recognized by the Nobel Prize committee as well, gave half of his monetary award to Best. Macleod then shared his with J.B. Collip, the chemist who had worked with them to purify insulin for clinical trials.
After continuing his studies in Canada, the United States, and Europe, Best returned to the University of Toronto in 1929 to become the head of the physiology department. He became director of the Banting-Best Department of Medicine Research after Banting's death in 1941.
3. J.B Collip (from McGill University)
http://www.mqup.mcgill.ca/book.php?bookid=1639
From a blurb for a book about the life and accomplishments of J.B Collip:
In the early years of the twentieth century medical research in Canada was the job of a select few. By mid-century it had grown into a systematic, large-scale venture that involved teams of professional scientists and dozens of laboratories in universities, government, and industry. J.B. Collip - skilled both as a bench scientist and an entrepreneur - played a leading role in this transformation. In J.B. Collip and the Development of Medical Research in Canada Alison Li details how Collip leapt into prominence in 1921-22 as part of the team at the University of Toronto that isolated insulin. When the Nobel Prize was awarded to Frederick Banting and J.J.R. Macleod in 1923, Banting announced he was sharing his award with Charles Best; Macleod in turn announced he was sharing his award with Collip.Collip was known for his remarkable skills in making hormone extracts, many of which proved to have therapeutic, and therefore commercial, value. At McGill University in the 1930s he headed a thriving research group that carried out investigations of the pituitary and sex hormones, including development of one of the first orally active estrogen products. Collip's story sheds light on early negotiations between academic science and the pharmaceutical industry and on the complexities of sustaining a research laboratory before the rise of government funding. As the head of the National Research Council's medical research division during its formative years, Collip helped shape the foundations of organized support for medical research in Canada.
4. J.J. Macleod (from PBS) http://www.pbs.org/wgbh/aso/databank/entries/dm22in.html
In 1920, Canadian surgeon Frederick Banting visited the University of Toronto to speak to the newly appointed head of the department of physiology, John J.R. Macleod. Macleod had studied glucose metabolism and diabetes, and Banting had a new idea on how to find not only the cause but a treatment for the so-called "sugar disease."Late in the nineteenth century, scientists had realized there was a connection between the pancreas and diabetes. The connection was further narrowed down to the islets of Langerhans, a part of the pancreas. From 1910 to 1920, Oscar Minkowski and others tried unsuccessfully to find and extract the active ingredient from the islets of Langerhans. While reading a paper on the subject in 1920, Banting had an inspiration. He realized that the pancreas' digestive juice was destroying the islets of Langerhans hormone before it could be isolated. If he could stop the pancreas from working, but keep the islets of Langerhans going, he should be able to find the stuff! He presented this idea to Macleod, who at first scoffed at it. Banting badgered him until finally Macleod gave him lab space, 10 experimental dogs, and a medical student assistant.
In May, 1921, as Macleod took off for a holiday in his native Scotland, Banting and his assistant Charles Best began their experiments. By August they had the first conclusive results: when they gave the material extracted from the islets of Langerhans (called "insulin," from the Latin for "island") to diabetic dogs, their abnormally high blood sugars were lowered. Macleod, back from holiday, was still skeptical of the results and asked them to repeat the experiment several more times. They did, finding the results the same, but with problems due to the varying purity of their insulin extract.
Macleod assigned chemist James Bertram Collip to the group to help with the purification. Within six weeks, he felt confident enough of the insulin he had isolated to try it on a human for the first time: a 14-year-old boy dying of diabetes. The injection indeed lowered his blood sugar and cleared his urine of sugars and other signs of the disease. Banting and Best published the first paper on their discovery a month later, in February, 1922. In 1923, the Nobel Prize was awarded to Banting and Macleod for the discovery, and each shared their portion of the prize money with the other researchers on the project.
Ironically, Banting's original idea wasn't entirely correct. He and Best later found they could obtain insulin even from an intact pancreas. Improved technology for testing and detecting sugar in the blood and urine provided information that earlier researchers didn't have, and this encouraged them to pursue a line of thinking that may have looked like a dead end to those working in the decades before them.
The discovery of insulin was one of the most revolutionary moments in medicine. Though it took some time to work out proper dosages and to develop manufacturing processes to make enough insulin of consistent strength and purity, the introduction of insulin seemed literally like a miracle. One year the disease was an automatic death sentence; the next, people -- even children -- had hopes of living full and productive lives even with the disease. Estimates show there are more than 15 million diabetics living today who would have died at an early age without insulin.
The Nobel Foundation’s decision to
award the prize to Banting and Macleod and to leave out Best and Collip
was controversial.
The following article from the web site of the Nobel Foundation Museum Association discusses it: August Krogh and the Nobel Prize to Banting and Macleod by Jan Lindsten http://www.nobel.se/medicine/articles/lindsten/
Here is an interesting factoid from: http://www.nobel.se/about/sponsors/index.html :
According to the rules of the Nobel Foundation, the interest of the funds of Alfred Nobel can only be used for the Nobel Prizes. All other activities, i.e. symposia, exhibitions and museums must be funded by external grants. The Nobel Foundation Museum Association, an organization affiliated with the Nobel Foundation, is responsible for the corporate sponsorship relations of Nobel e-Museum.
Other Links:
1. Diabetes FAQ
http://www.faqs.org/faqs/diabetes/
2. Timeline of the history of diabetes
from the Canadian Diabetes Association:
http://www.diabetes.ca/Section_About/timeline.asp
3. Diagnostics in Healthcare (The
newsletter of the British In Vitro Diagnostics Association)
http://www.bivda.co.uk/Document/DocumentDownload.cfm/dih%204.pdf?DType=DocumentItem&Document=dih%204.pdf